Leveraging a Proven Mechanism with Data-Driven Approach to Bolster Development for a Broad Range of Indications.
LB-102 is a new chemical entity and a methylated derivative of amisulpride, a second-generation antipsychotic drug approved in over 50 countries, not including the United States.
History of Amisulpride
Amisulpride has been extensively used in clinical practice following its initial approval in France in the 1980s, generating at least two million monthly prescriptions in 2023 in a select group of European counties including France, Germany, Italy, Spain and others. Among these European prescriptions for amisulpride, approximately 60% are for schizophrenia and schizoaffective disorders, approximately 20% are for mood disorders, approximately 14% are for anxiety, and the remainder are for a variety of other indications.
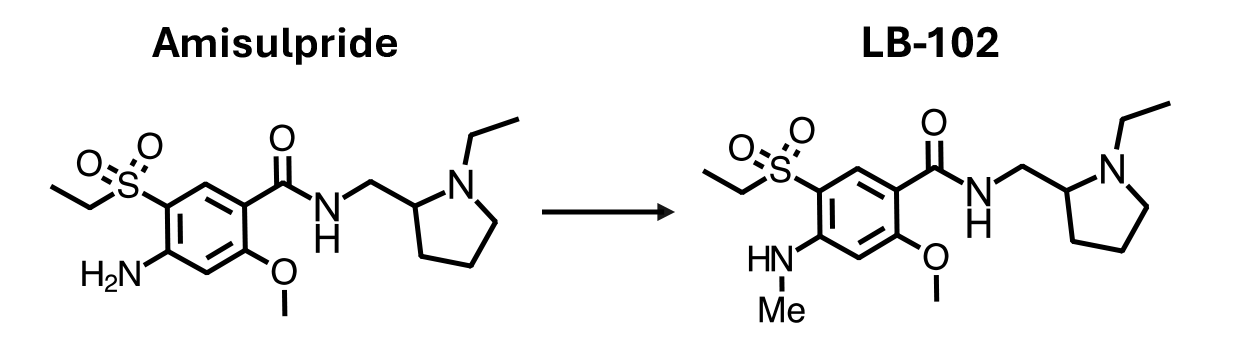
Creation of LB-102
LB-102 is a novel benzamide antipsychotic in development for the treatment of schizophrenia. Developed to address key limitations of both amisulpride and existing therapies, LB-102 builds on the chemical foundation of amisulpride with a subtle structural modification—a single methyl group—that enhances its ability to cross the blood-brain barrier while preserving receptor potency and selectivity. This targeted change enables once-daily oral dosing and allows LB-102 to be administered at lower doses than twice-daily amisulpride, with the goal of minimizing side effects, improving clinical results, and treatment adherence for patients.
Long-Acting Formulation Development
We are investigating alternate long-acting injectable (LAI) formulations of LB-102 for its potential to provide improved clinical results and treatment adherence for schizophrenia and bipolar depression patients compared to oral formulations, for which compliance may be lower. Additionally, an LAI formulation of LB-102, if approved, could have additional advantages, including extending the commercial protection for LB-102, potentially providing a better alternative to the currently limited option set of approved LAI antipsychotic drugs for other neuropsychiatric disorders, such as bipolar disorder or Alzheimer’s psychosis, and potentially enhancing LB-102’s competitive positioning outside of the United States.


