LB-102 is supported by its mechanism of action, positive Phase 2 data in acute schizophrenia, and the established clinical experience of amisulpride, providing a strong foundation for continued development in schizophrenia and bipolar depression.
About Schizophrenia
Prevalence of Schizophrenia
Typically diagnosed in the late teen years to early thirties, schizophrenia is a chronic, severe, complex, and debilitating psychiatric disorder that affects approximately 1% of the U.S. population and is a leading cause of disability.
Symptoms of Schizophrenia
Psychotic or positive symptoms
- Hallucinations
- Delusions
- Thought disorder
- Movement disorder
Negative symptoms
- Lack of motivation, interest, or enjoyment in daily activities
- Withdrawal from social life
- Difficulty in showing emotions
Cognitive symptoms
- Problems with attention, concentration, and memory
Current Treatments for Schizophrenia
While antipsychotic drugs are commonly used to treat schizophrenia, they fall short of the ideal profile, as most currently approved medications have significant side effects, primarily address positive symptoms of schizophrenia, and are often not effective in treating negative and cognitive symptoms.
LB-102 in Schizophrenia (NOVA1)
In January 2025, we announced positive data from a four-week placebo-controlled, double-blinded, Phase 2 trial in the United States, which assessed the safety and efficacy of LB-102 in patients with acute schizophrenia. Results from the trial demonstrated a statistically significant clinical activity at all LB-102 doses tested, a significant average change in overall symptoms, a potentially class-leading tolerability profile among D2/D3 antagonists and partial agonists, and a potentially differentiated impact on cognition (measured by CogState Computerized Schizophrenia Battery of Tests).
The trial achieved its primary endpoint of change in the Positive and Negative Syndrome Scale (PANSS), a 30-item scale that measures the severity of schizophrenia symptoms, from baseline to Week 4. A statistically significant decrease in symptoms was observed for all three dose cohorts (50 mg, 75 mg, and 100 mg) compared to placebo. Additionally, our Phase 2 trial data showed a statistically significant impact on negative symptoms versus placebo at the 50 mg dose even though the inclusion criteria enriched for patients experiencing predominantly positive symptoms of schizophrenia. The impact of LB-102 on cognitive function was also evaluated as an exploratory endpoint in this trial. After four weeks of treatment with LB-102, a robust, dose-dependent, and significant treatment effect size was identified in a post-hoc analysis in the completer population for all doses of LB-102 compared with placebo.
LB-102 was generally well tolerated in the clinical trial, with adverse events being mostly transient and mild to moderate in severity. The most commonly reported treatment emergent adverse events were psychiatric neurological conditions that many participants were experiencing at the time of enrollment including insomnia, headache, anxiety and agitation. Because of the frequency of certain adverse events associated with schizophrenia medications, we identified adverse events of special interest, including extrapyramidal symptoms (EPS), sedation, adverse events associated with a prolactin increase, and QT prolongation. Rates of EPS (including akathisia) ranged from 0.9-5.6% in the LB-102 treated arms versus 3.7% in the placebo group. There was a single case of sedation (75 mg) among 251 patients exposed to LB-102 compared with none in placebo. Adverse events related to increase in prolactin occurred in 0.9-5.6% of patients treated with LB-102 compared with none in placebo; and the mean change in QT from baseline ranged from 4.3-5.4 ms compared with 1.7 ms with placebo. No patients met the prespecified stopping criteria with respect to QT prolongation.
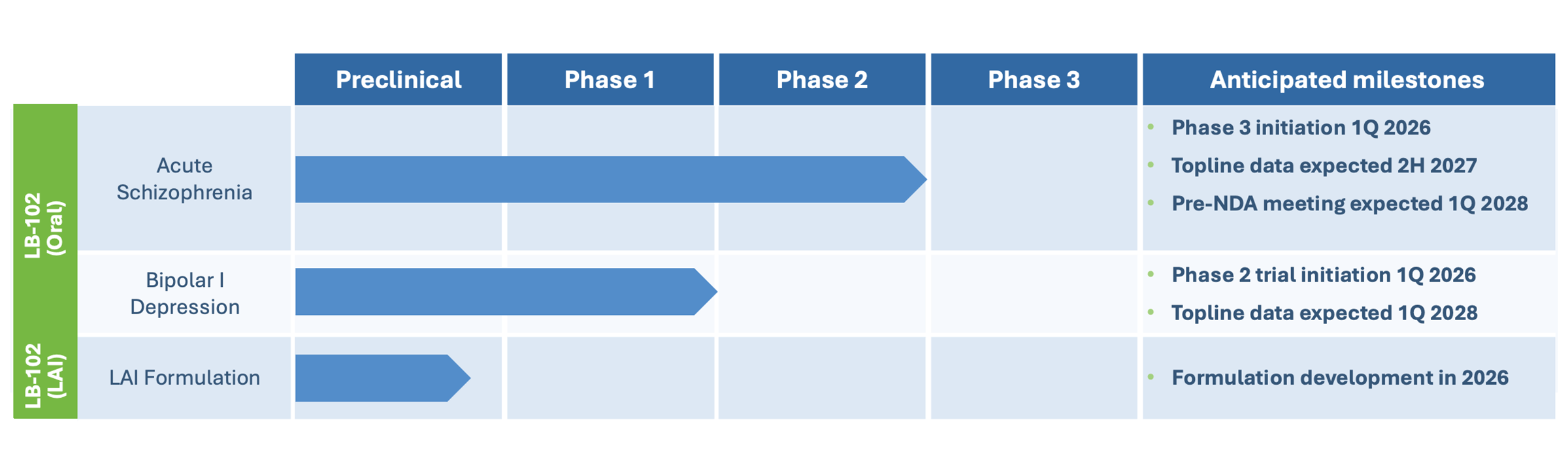
These results have informed the design of a six-week Phase 3 trial in acute schizophrenia, which we aim to commence in the first quarter of 2026. If positive, we believe the data could be sufficient to support a regulatory application for approval in the United States along with our completed Phase 2 trial and other planned NDA-enabling studies.
About Bipolar Depression
Prevalence of Bipolar Depression
It is estimated that 2.8%, or approximately seven million Americans, experience bipolar disorder in a year, and approximately 40 million people live with bipolar disorder worldwide.
Symptoms of Bipolar Depression
Most people living with bipolar depression experience dramatic shifts in mood, energy, and behavior, alternating between manic and depressive states.
Current Treatments for Bipolar Depression
While recovery with proper treatments such as mood stabilizers and antipsychotic drugs is possible, unmet needs remain. Antipsychotics are a mainstay of treatment for bipolar depression due to their ability to alleviate depression while simultaneously preventing the emergence of mania. Unmet needs include therapies that offer fewer adverse events, better efficacy, including improvements in cognition and anhedonia, improved compliance to treatment, and a desire for therapies with novel mechanisms of action.
About LB-102 in Bipolar Depression
We believe there is strong scientific and clinical rationale for development of LB-102 in the treatment of depressive episodes associated with bipolar disorder or bipolar depression (BPD). LB-102’s mechanism, characterized by potent and selective antagonism of D2, D3, and 5HT7 receptors, may offer dual therapeutic benefits, controlling psychosis and mania via D2 blockade and potentially enhancing antidepressant and cognitive effects through antagonism of 5HT7 and D3.
In a Phase 2 trial in acute schizophrenia, LB-102 demonstrated strong antipsychotic activity and a generally favorable tolerability profile, including low rates of extrapyramidal symptoms, sedation, and gastrointestinal side effects. The cognitive findings observed in this study further suggest LB-102 may hold potential for differentiating in BPD. Amisulpride is approved in certain European countries for dysthymia, a form of depression, and has been shown to be as effective as certain approved agents for MDD. We believe that results in dysthymia and MDD provide strong scientific and clinical rationale for development of LB-102 in the treatment of depressive episodes associated with bipolar disorder because episodes of major depression, whether unipolar (as in MDD) or bipolar (as in bipolar depression), are typically characterized by a similar imbalance in the neurotransmitters serotonin, noradrenaline, and dopamine, regardless of the underlying pathophysiology of the disease.
Our initial Phase 2 trial will explore the utility of LB-102 in controlling the depressive symptoms of the disease. We plan to initiate this potentially registrational Phase 2 trial in bipolar depression in the first quarter of 2026, with topline data expected in the first quarter of 2028.
Additional Neuropsychiatric Diseases
Pending successful development in BPD, LB-102 may also be explored as a potential treatment for major depressive disorder (MDD). Additional areas of future investigation could include neuropsychiatric conditions such as schizophrenia with predominantly negative symptoms, Alzheimer’s disease-related agitation and psychosis, manic episodes associated with bipolar disorder, and cognitive impairment associated with schizophrenia, or CIAS.
LB Pharmaceuticals believes that if approved, LB-102 has the potential to serve as a mainstay of psychiatric practice by offering a potentially attractive alternative to branded and generic therapeutics for the treatment of schizophrenia, bipolar depression, and other neuropsychiatric diseases.


